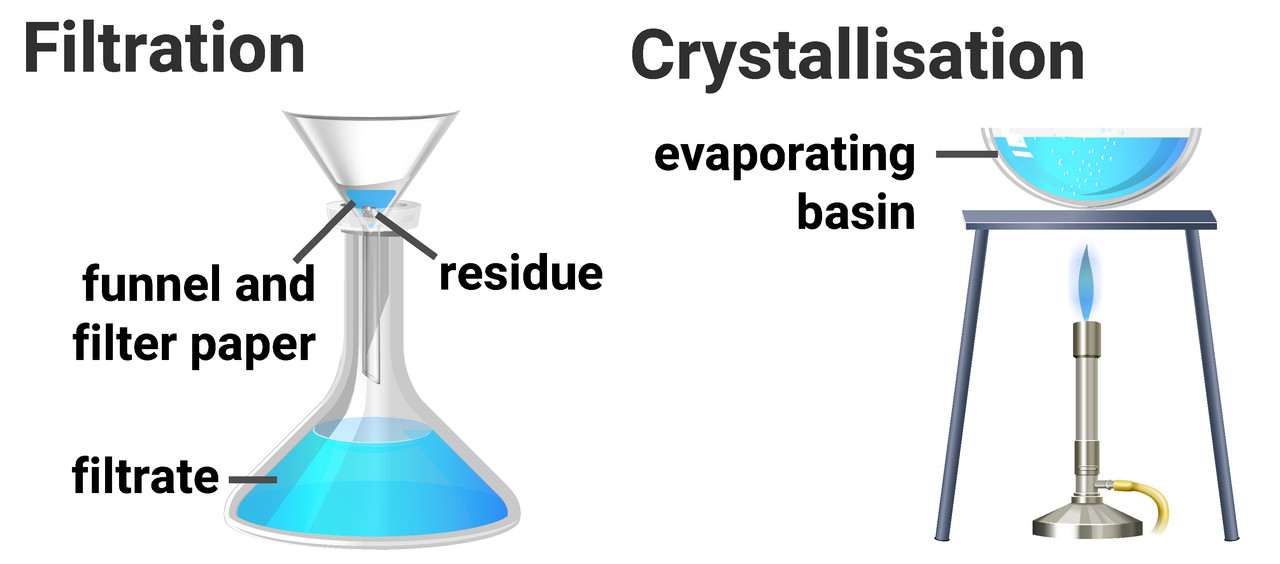
There is an ongoing debate in school districts around the world on the notion of streaming high performing students into different classes based on their performance. On the one hand, this offers teachers a more homogenous group of students to work with, hopefully ensuring that the level of instruction is appropriate for all students in the classroom but, on the other hand students suffer serious real-world consequences from being placed in ‘low’ performing classes. The crux of this argument lands in “quantification” and efficiency, so let’s make a chemistry comparison and see what we find!
If we have an amount of liquid water and we want to turn it into gaseous water, we can! If we were to leave the water in a class, on the counter, it would evaporate eventually. We can change parameters to increase the rate of evaporation. We could:
- Add heat to the water, increasing the energy of the system and converting them to gas.
- Add heat to the room*; this might seem repetitive but consider that a counter in Canada in December is colder than a counter in Cuba in June
- Remove water from the atmosphere, reducing the humidity will encourage water uptake by the atmosphere.
- change the shape of the container; a shallow wide bowl will evaporate quicker than a tall thin drinking glass.
- Separate the water into more containers, increasing the surface area.
There are definitely more options available to us, but this is sufficient for now. Notice how we can make efforts on the water, but also on the environment, and the structure of the water. This is comparable to the students’ position. Achievement in a class can be directed to them. We can give them one on one time with the teacher or tutoring to encourage their development. We can also make changes to the climate of education by considering how many friends and bullies are in the class or how engaged is the student. Finally structures and pressures of education exist. Maybe a student has driven academic parents, or they have aloof parents who treat education like a day care. Did you notice we asserted that the water should be gaseous in this example? Why didn’t we consider the inverse; that the water should be frozen, or maybe left as water and preserved? This value-based assertion occurs in every school and often without consulting the student on their desired outcome.
Consider now that we want to boil all the water out of a bottle of Sprite*. We could do it slowly, or quickly and again we’re looking to boil off the water. The first gas will come off as soon as you crack the lid. Carbon dioxide will fizz out of the bottle and left unattended will escape pretty quickly. If you wait the water will evaporate, so adding heat will boil it quickly. Hold on! What about the sugars in the bottle? No matter how much heat you add to the system they will not evaporate into gaseous sugars. They will crystalize, combust and break down depending on the intensity and duration of the heat. In a non-homogenous class (regular class room) it’s likely that anyone strategy will benefit some, but harm others.
For me this is where the argument for classroom streams is strongest. It’s efficient and simplifies the work that teachers, admins and post-secondary institutions need to do to evaluate people, but it’s not the best system for each individual. It’s cold, it’s industrial, and its quantifiable but it’s not true.

It’s important now to drop the chemistry analogy. As much as we’d like to simply and reduce, humans are simply more complicated than chemical mixtures. Maybe one day in the future, a powerful AI with a true perfect Lense into our every waking moment, we will be able to reduce the actions of humanity to the physics that govern billiards, but until then we need to consider the human element.
Humans do the following that atoms do not
- care for each other; friends and family are invested in the outcomes of specific individuals
- communicate using language; Objectively, the languages spoken by humans have different properties than the sharing of electrons between atoms.
- form connections: While atoms and compounds ca re-arrange into man different shapes and forms it’s still important to observe, they are not the kinds of connections formed by communities.
- Emote; We’re emotional beings! One day someone could be happy and the next, depressed or angry. We even change within a few seconds (situation depending)
Recognizing these complexities to the human element is the first step to additional solutions for the problem of “streaming students”. These human solutions to streaming will be explored in my next post.